Electron extended states in proteins
Review of the studies carried out
Localized extended electron states (polarons, excitons and F-centers) play an important role in the solid state physics. A great stride towards providing the groundings for the molecular electronics theory is the study of the electron properties of individual molecules. The knowledge of these properties can be used as the basis for the theory of electron transfer in molecular complexes.
The IMPB RAS has developed a new approach to the problem of electron states in protein macromolecules. Most investigations have been centered on modeling of weakly-coupled excess electrons in metal containing proteins of the electron transport chain (azurines, plastocyanides, cytochromes). Such proteins generally occur in water surroundings. Protein medium, being much less polar than water, is much more polar, than most of ion crystals, dealt with in the solid state physics. Under these conditions electron states are not simply modified by the nearest surroundings, but take on a new quality, changing from localized states to large-radius extended states. This brings up a need to solve the bound polaron state problem in a spatially heterogeneous medium. Within the simplest model of “dielectric cavity” the spatial heterogeneity is simulated by a spherical region with dielectric permittivity distinct from that of the solvent. Fig.1 shows the electron density distribution for a three-layer model of a protein globule. In a solution such extended states are comparable in size with the globule.
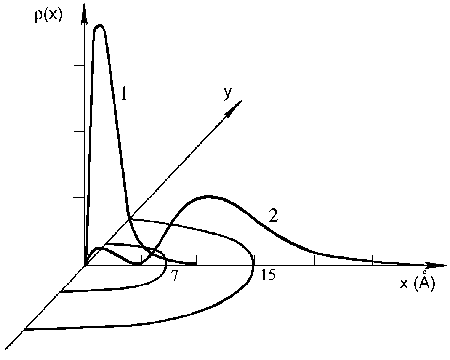
Fig.1. Electron density distribution in a protein globule:
1 – zero mode, 2 – the first mode